Back to menu
View all Our Products
Cleaning
Chemicals
Paper
Waste
Machines
Workwear PPE
Facilities Supplies
Skincare
Back to menu

Cleaning & Hygiene Products
We stock thousands of high-quality branded and own-brand cleaning and hygiene products ready to tackle any cleaning tasks

Account Management Platform
Manage your orders, finances, product collections, sites and users from a single online dashboard.

Easy Access QR Code Ordering
Allow staff to order pre-approved products and complete administrative tasks from a simple on-site poster.

Windmill Refill
Exclusive range of cleaning chemicals delivered in reusable bottles for collection, refill and redistribution

Digital Dosing Charts Generator
Making your cleaning processes smarter, safer, and more profitable

Free COSHH Training
Be safe and compliant with our free online COSHH training and assessment platform.

Customer Care
Friendly and knowledgeable staff on hand to support you with your cleaning and hygiene needs

Environmental & Sustainability
We are dedicated to creating a more sustainable world for our customers and community.
Back to menu

Contract Cleaners
We supply high-quality cleaning and hygiene products to cleaning contractors across the UK.

Education
We work with schools, colleges and universities to ensure their environments remain clean and sanitary.

Leisure & Tourism
Helping to keep the places clean when people like to stay and play

Public Sector
Keeping public spaces and building clean and hygienic for everyone to enjoy.

Healthcare
Supporting healthcare facilities with certified products creating clean, sanitary and compliant spaces.

Food Manufacturers
Many of our cleaning chemicals are food safe and ideal for food manufacturing facilities

Commercial Facilities
Supporting Contract Cleaners and Facilities Managers in keeping their offices and commercial spaces clean and tidy

Industrial
Reliable cleaning products and support services for demanding, high-performance industrial environments.
Back to menu

Windmill Refill
Windmill is our own-range of high-quality cleaning products with Refill and Eco options available.

Tork
Paper products and dispenser from leading UK brand Tork

Raphael
Toilet rolls, hand towels, jumbo rolls and dispenser from Raphael

MotorScrubber
Industry leading floor cleaning machines from MotorScrubber

Rubbermaid
Waste management solutions suitable for commercial environments

Numatic
High performance and sustainable cleaning machines from Numatic

RugDoctor
Great value and easy-to-use carpet cleaning machines and accessories from RugDoctor

Truvox
Commercial and Industrial floorcare machines and accessories from Truvox

Prochem
Professional-grade cleaning products and equipment engineered for exceptional results in commercial spaces
Back to menu
Back to categories
View all Cleaning
Cloths, Dusters and Scourers
Mopping
Buckets, Caddys and Containers
Brushware
Cleaning Trolleys
Window Cleaning
High Level Cleaning
Chemical Dispensing and Sprayers
Scrapers
Wipes
Wall Brackets
Back to Cleaning
View all Cloths, Dusters and Scourers
Microfibre Cloths
All Purpose Cloths
Stockinette Cloths
Scourers and Sponges
Dusters
Tea Towels
Rags
Back to Cleaning
View all Mopping
Mop Buckets
Exel Socket Mop System
Hygiemix Screw In Mop System
Kentucky Mop System
Interchange Mop System
Vileda Mop System
Rubbermaid Hygen Mop System
Smart Mop System
MotorScrubber Blade Mop System
SYR Flat Mop System
Sponge Mop System
AUK Pro Flat Mop System
Squeegees
Numatic Flat Mop System
Back to Cleaning
View all Buckets, Caddys and Containers
Buckets and Lids
Cleaning Caddys
Containers
Back to Cleaning
View all Brushware
Toilet Brushes
Dustpans and Brushes
Wooden Brush Heads
Plastic Brush Heads
Complete Brooms
Hand and Specialist Brushes
Plastic Hand Brushes
Tube Cleaning Brushes
Handles
Cleaning Pads and Holders
Dish Brushes
Nail Brushes
Edging Tools
Straight Sweepers
Grout Brushes
Vehicle Brushes
Carpet and Upholstery Brushes
Floor Squeegees
Back to Cleaning
View all Window Cleaning
T-Bars and Sleeves
Squeegees and Rubbers
Buckets, Poles and Holders
Pure Water Systems
Window Cleaning Accessories
Microfibre Internal Window Cleaning
Back to categories
View all Chemicals
Windmill Refill
Washroom Chemicals
Hard Floor Chemicals
Hard Surface Cleaners
Carpet Chemicals
Kitchen Chemicals
Laundry Chemicals and Powders
Bleach, Disinfectants and Sanitisers
Air Care
Problem Solving Chemicals
Branded Chemicals
Chemical Dispensing and Sprayers
Automotive
Back to Chemicals
View all Washroom Chemicals
Toilet Cleaners and Descalers
Washroom Surface and Floor Chemicals
Urinal Deodorant Blocks, Mats and Clips
Toilet Seat Sanitisers
Back to Chemicals
View all Hard Floor Chemicals
Hard Floor Cleaning Chemicals
Scrubber Drier Chemicals
Floor Polish Strippers
Floor Seals and Polishes
Polished Floor Maintainers
Back to Chemicals
View all Hard Surface Cleaners
Multipurpose Cleaners
Glass Cleaners
Furniture Polish
Metal Cleaners
Back to Chemicals
View all Carpet Chemicals
Extraction Cleaner Chemicals
Presprays and Traffic Lane Cleaners
Spot and Stain Removers
Deodorisers and Sanitisers
Protective Treatments
Rug Doctor System
Encapsulating System
Bonnet Buffing Products
Dry Cleaning Products
Upholstery and Fine Fabric Chemicals
Leather Cleaners
Accessories
Back to Chemicals
View all Kitchen Chemicals
Washing Up Liquid
Degreasers
Sanitisers
Destainers
Dishwash Chemicals
Dishwashing Salt
Oven Cleaners
Descaler
Back to Chemicals
View all Laundry Chemicals and Powders
Laundry Liquids
Laundry Powders
Laundry Tablets and Pods
Back to Chemicals
View all Air Care
Air Freshener Aerosols
Air Fresheners for Dispensers
Air Freshener Dispensers
Air Freshener Solid Gels
Deodorisers and Odour Neutralisers
Air Freshener Kits
Aerosol Sanitisers
Back to Chemicals
View all Problem Solving Chemicals
Chewing Gum Removal
Graffiti Removal
Label Removal
Lubricants
Drain Cleaners
Descalers
Mould Removing Chemicals
Waste Tank Defoamers
Back to Chemicals
View all Branded Chemicals
Clover Super Dose Concentrates
Selden Ecoflower
PVA Hygiene Sachets & Bottles
Back to Chemicals
View all Chemical Dispensing and Sprayers
Trigger Sprays
Dosing Pumps and Taps
Jugs and Funnels
Pressure Sprayers
Back to categories
View all Paper
Toilet Roll
Hand Towels
Blue Roll
Couch Rolls
Kitchen Roll
Wiper Roll
Facial Tissues
Toilet Roll Dispensers
Rolled Hand Towel Dispensers
Folded Hand Towel Dispensers
Couch Roll Dispensers
Dispenser Keys
Back to Paper
View all Toilet Roll
Standard Toilet Rolls
Mini Jumbo Toilet Rolls
Jumbo Toilet Rolls
Bulk Pack Toilet Rolls
Coreless Toilet Rolls
Raphael Toilet Rolls
Leonardo Toilet Rolls
Katrin Toilet Rolls
Tork Toilet Rolls
Lucart L-ONE Toilet Rolls
Scott Toilet Rolls
Back to Paper
View all Toilet Roll Dispensers
Plastic Dispensers
Metal Dispensers
Leonardo Dispensers
Tork Dispensers
Raphael Dispensers
Katrin Dispensers
Lucart Dispensers
Back to Paper
View all Rolled Hand Towel Dispensers
Metal Dispensers
Plastic Dispensers
Raphael Dispensers
Tork Dispensers
Back to Paper
View all Folded Hand Towel Dispensers
Metal Dispensers
Plastic Dispensers
Tork Dispensers
Katrin Dispensers
Back to categories
View all Waste
Sacks, Bags and Bin Liners
Recycling
Rubbermaid Slim Jim Bins
Rubbermaid Brute Bins
Outdoor Bins
Swing Top and Open Top Bins
Sanitary Bins
Sharps Disposal
Litter Pickers
Back to Waste
View all Sacks, Bags and Bin Liners
Bin Liners
Black Sacks
Clear Sacks
Coloured Sacks
Compactor Sacks
Wheelie Bin Liners
Clinical Waste Sacks
Compostable Bags
Carrier Bags
Rubble Sacks
Back to categories
View all Machines
Vacuum Cleaners
Rotary Floor Machines
Scrubber Dryers
Carpet Cleaners
Hard Surface Cleaners
Pressure Washers and Sprayers
Sweepers and Litter Collectors
Foggers
Carpet and Room Dryers
Floor Pads
Spares and Accessories
Chewing Gum Removers
Back to Machines
View all Vacuum Cleaners
Dry Vacuum Cleaners
Backpack Vacuum Cleaners
Upright Vacuum Cleaners
Wet and Dry Vacuum Cleaners
Advanced Filteration Vacuum Cleaners
Industrial Vacuum Cleaners
Back to Machines
View all Floor Pads
Standard Speed Floor Pads
Ultra-High Speed Floor Pads
Diamond Floor Pads
Melamine Deep Clean Floor Pads
Sandscreen Sanding Pads
Back to Machines
View all Spares and Accessories
Dust Bags
General
Craftex
Denis Rawlins
Fimap
IVO
Kärcher
Mastervac
MotorScrubber
Nilfisk
Numatic
Osprey
Pacvac
Prochem
Rug Doctor
Sebo
Truvox
Back to categories
View all Workwear PPE
Clothing
Footwear
Hi-vis Clothing
Rainwear
Eye and Face Protection
Face Masks
Head Protection
Ear Protection
Gloves and Sleeves
Knee, Elbow and Shoulder Pads
Harnesses and Straps
Disposable Outerwear
Back to Workwear PPE
View all Clothing
Rugby and Polo Shirts
Vests, Overalls, Bibs and Braces
Jackets, Coats and Bodywarmers
Hoodies, Fleeces and Sweatshirts
T-Shirts and Shirts
Trousers, Shorts and Cargo Pants
Tabards, Tunics and Aprons
Thermal and Base Layer Clothing
Catering Clothing
Ties and Scarves
Maternity Clothing
Back to Workwear PPE
View all Footwear
Boots
Shoes
Trainers
Wellingtons and Waders
Accessories
Rigger Boots
Safety Shoes
Safety Boots
Safety Trainers
Steelite Boots
Steelite Shoes
Derby Boots
Hiking Boots
Socks
Back to Workwear PPE
View all Hi-vis Clothing
Hi-Vis Coats and Jackets
Hi-Vis Vests and Bodywarmers
Hi-Vis Polo Shirts
Hi-Vis Fleeces, Jumpers and Sweatshirts
Hi-Vis Footwear and Gloves
Hi-Vis Trousers
Hi-Vis Coveralls
Hi-Vis Shirts and T'shirts
Hi-Vis Bib and Braces
Hi-Vis Shorts
Hi-Vis Flame Resistant Clothing
Hi-Vis Rainsuits
Hi-Vis Base Layers
Hi-Vis Hats and Beanies
Back to Workwear PPE
View all Rainwear
Waterproof Suits
Waterproof Jackets
Waterproof Trousers
Waterproof Hats and Gloves
Back to Workwear PPE
View all Eye and Face Protection
Safety Spectacles
Safety Goggles
Lens Cleaning
Face Visors
Back to Workwear PPE
View all Face Masks
Disposable Moulded Masks
Disposable V-Fold Masks
Full and Half Masks
Mask Filters
Washable Masks
Back to Workwear PPE
View all Head Protection
Mob Caps and Skull Caps
Safety Helmets
Accessories for Safety Helmets
Baseball and Bump Caps
Hats, Beanies and Balaclavas
Back to Workwear PPE
View all Gloves and Sleeves
Handling Gloves
Disposable Gloves
Inspection and Rigger Gloves
Chemical Resistant Gloves and Gauntlets
Cut Resistant Gloves and Sleeves
Heat Resistant Gloves and Gauntlets
Needlestick Resistant Gloves and Gauntlets
Specialist Handling Gloves
Latex and Nitrile Gloves
Fingerless, Winter and Driving Gloves
Cuffs and Sleeves
Rubber Gloves
Back to Workwear PPE
View all Disposable Outerwear
Hair Nets
Over Sleeves
Aprons
Over Shoes
Overalls
Beard Snood
Dispensers
Back to categories
View all Facilities Supplies
First Aid
Safety
Signage
Catering
Washroom
Outdoor Equipment
Electrical
Door Hardware and Security
Pest Control and Weedkiller
Business Supplies
Automotive
Bags
Household
Back to Facilities Supplies
View all First Aid
Burns Kits and Accessories
Defibrillators and Accessories
Eye Wash
First Aid Equipment
First Aid Kits
First Aid Room Essentials
Plasters, Dressings and Tapes
Skin Wipes and Antiseptics
Back to Facilities Supplies
View all Safety
Fire Blankets
COSHH Cabinets
Body Fluid Spill Kits
Maintenance Spill Control
Chemical Spill Control
Screen Guards
Traffic Cones
Entrance Mats
Assistance
Back to Facilities Supplies
View all Signage
Fire Safety Signs
First Aid Signs
Health and Safety Signs
CCTV Signs
Bespoke Signage
Back to Facilities Supplies
View all Catering
Equipment
Sink Essentials
Food Packaging
Consumables
Napkins and Tableware
Disposable Cutlery and Cups
Thermometers
Wrapmaster
Oven Cloths
Tea Towels
Greaseproof Bags
Back to Facilities Supplies
View all Washroom
Baby Changing Units
Toilet Seats
Feminine Hygiene
Hand Driers
Back to Facilities Supplies
View all Outdoor Equipment
Gardening
Ashtrays
Drain Clearing
Rock Salt and Spreaders
Shovels
Trucks and Carts
Grit Bins
Back to Facilities Supplies
View all Electrical
2D Light Bulbs
Extension Leads
Light Bulbs
Radiators
Small Electrical Appliances
Appliance Accessories
Back to Facilities Supplies
View all Door Hardware and Security
Door Stops and Wedges
Hinges
Finger Protectors
Door Closers
Door Handles and Locks
Padlocks
Signage
Security
Push Bars
Back to Facilities Supplies
View all Household
Candles and Fire Lighters
Knives and Blades
Ladders and Step Stools
Laundry
Bathroom Textiles
Batteries and Torches
Furniture
Back to categories
View all Skincare
Windmill Touch Hand Care
5L and 10L Products
Pump Tops
Diversey Products
Evans Products
Gojo Products
Katrin Products
Kimberly Clark Products
Raphael Products
Rubbermaid Products
Soft Care Products
SC Johnson Deb Products
Tork Products
Dispensers
Wipes
Medi9 Products
Back to Skincare
View all Dispensers
Plastic
Metal
Touch
Evans
Excel
Rubbermaid
SC Johnson Deb
Tork
Keys
Replacement Parts
Stands

Cleaning & Hygiene Products
We stock thousands of high-quality branded and own-brand cleaning and hygiene products ready to tackle any cleaning tasks

Account Management Platform
Manage your orders, finances, product collections, sites and users from a single online dashboard.

Easy Access QR Code Ordering
Allow staff to order pre-approved products and complete administrative tasks from a simple on-site poster.

Windmill Refill
Exclusive range of cleaning chemicals delivered in reusable bottles for collection, refill and redistribution

Digital Dosing Charts Generator
Making your cleaning processes smarter, safer, and more profitable

Free COSHH Training
Be safe and compliant with our free online COSHH training and assessment platform.

Customer Care
Friendly and knowledgeable staff on hand to support you with your cleaning and hygiene needs

Environmental & Sustainability
We are dedicated to creating a more sustainable world for our customers and community.

Contract Cleaners
We supply high-quality cleaning and hygiene products to cleaning contractors across the UK.

Education
We work with schools, colleges and universities to ensure their environments remain clean and sanitary.

Leisure & Tourism
Helping to keep the places clean when people like to stay and play

Public Sector
Keeping public spaces and building clean and hygienic for everyone to enjoy.

Healthcare
Supporting healthcare facilities with certified products creating clean, sanitary and compliant spaces.

Food Manufacturers
Many of our cleaning chemicals are food safe and ideal for food manufacturing facilities

Commercial Facilities
Supporting Contract Cleaners and Facilities Managers in keeping their offices and commercial spaces clean and tidy

Industrial
Reliable cleaning products and support services for demanding, high-performance industrial environments.

Windmill Refill
Windmill is our own-range of high-quality cleaning products with Refill and Eco options available.

Tork
Paper products and dispenser from leading UK brand Tork

Raphael
Toilet rolls, hand towels, jumbo rolls and dispenser from Raphael

MotorScrubber
Industry leading floor cleaning machines from MotorScrubber

Rubbermaid
Waste management solutions suitable for commercial environments

Numatic
High performance and sustainable cleaning machines from Numatic

RugDoctor
Great value and easy-to-use carpet cleaning machines and accessories from RugDoctor

Truvox
Commercial and Industrial floorcare machines and accessories from Truvox

Prochem
Professional-grade cleaning products and equipment engineered for exceptional results in commercial spaces
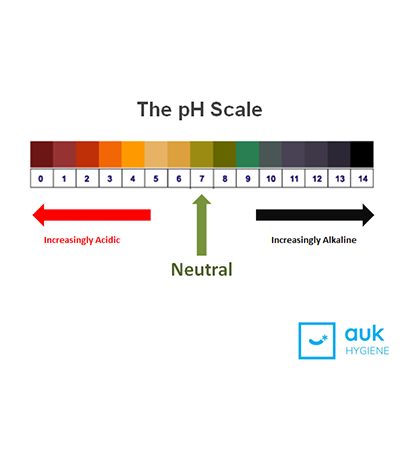
The pH Scale
10 May 2022
You learnt about the pH scale at school all those years ago, and you probably listened with half an ear, right? Well, you need to know about it because it applies when you are choosing a chemical to clean with. This knowledge will keep you safe, but also explain how effective the product will be to use.
This knowledge will keep you safe, but also explain how effective the product will be to use.
The science bit, what does it mean?
The ‘pH’ bit is an abbreviation of ‘potential of hydrogen’, and the numbers on the scale represent the concentration of hydrogen ions in a water-based solution. A high pH is a higher concentration, and a low pH scale is a lower concentration, or even an absence of hydrogen altogether.
Running from 0-14, the pH scale can help you to determine whether a substance is acidic or alkaline. A substance with a pH of 7 is neutral; anything lower than 7 is increasingly acidic, with 0 being the most acidic, and anything higher than 7 is increasingly alkaline, with 14 being the most alkaline. In a neutral solution you have both acidic and alkaline elements but they are equal making the product neutral.
Next, there is one other factor relative to pH that needs to be explained, and has to do with the corrosivness of a compound. Both acids and alkali’s have the capability of being corrosive, to classify a product or compound as being corrosive means that it would have the potential to eat eaway at something, in some cases very rapidly, and it would have the capability of being harmful to human tissue as well as inanimate objects.
In a neutral solution you have both acidic and alkaline elements but they are equal making the product neutral.
Knowledge is Power When it Comes to Effective Cleaning
The pH scale is always marked on the COSHH sheet and often on the label of products and you should check this before using.
It is therefore essential that the correct product is selected for the task at hand and it is correctly diluted. If you are still confused or would like further help do not hesitate to contact the Customer Service team on 01626 355177.
How Does the pH Scale Relate To Cleaning Chemicals?
When cleaning a substance off a hard surface or a carpet, your goal is to neutralize the soil’s acidic or alkaline ions. Acid neutralizes alkaline, and alkaline neutralizes acid. Therefore, contrary to the myth that a higher pH level makes the best cleaning product.
Your goal is to neutralize the soil’s acidic or alkaline ions.
The following is actually true:
- To clean acidic soils, you want a cleaning product that falls in the alkaline spectrum (pH greater than 7)
- To clean alkaline soils, you want a cleaning product that falls in the acidic spectrum (pH less than 7)
For acid-based soils, such as those listed below, use alkali cleaners.
- Greasy floors
- Dirty walls
- Cigarette tars
- Engines and tools
- Motor oils, diesel oil, axle grease
- Cooking oil
- Ventilation hoods
- Ovens
For alkaline-based soils, such as those listed below, use acid cleaners.
- Water spots
- Rust
- Calcium deposits
- Lime deposits
- Inside the dishwasher
- Toilet bowls
- Shower stalls
- Urinals
It is important that every one who uses cleaning chemicals understands that some compounds are corrosive by their very nature and should be handled according to the label directions on the container. Any corrosive chemical will have the corrosive symbol on the container
All corrosive products are labeled as such and must follow strict guidelines. This also shows that the incorrect use of chemicals can damage surfaces when cleaning. When you understand the proper use of cleaning chemicals, it becomes increasing clear that knowing about pH is pretty important!
Want to know more? Contact our specialist Customer Service team today
Featured articles



Don’t have enough time in the day to do everything yourself?
Why not give us a call and see how we can help you with your needs?
Connect
© Copyright 2026 AUK Hygiene. All rights reserved.
This website uses cookies to ensure you get the best experience. Learn more
